 back back |
 up up |
||||||||
 |
 |
||||||||
| Written By: Kambiz Nazridoust | |||||||||
| Introduction | |||||||||
|
Gas hydrate(clathrate) is an important topic for study for three reasons: 1. It contains a great volume of methane, which indicates a potential as a future energy resource When hydrate fills the pore space of sediment, it can reduce permeability and create a gas trap. Such trapping of gas beneath hydrate may cause the formation of the most concentrated hydrate deposits, due to the presence of a reservoir of gas below the hydrate zone. The gas can continually migrate upwards to fill any open pore spaces. This process, in turn, causes the trap to become more effective, producing highly concentrated methane and methane hydrate reservoirs. Gas from hydrate might become a major energy resource if economically profitable techniques could be devised to extract its methane. 2. It may function as a source or sink for atmospheric methane, which may influence global climate Methane from the hydrate reservoir might significantly modify the global greenhouse, because methane is ~20 times as effective a greenhouse gas as carbon dioxide, and gas hydrate may contain three orders of magnitude more methane than exists in the present-day atmosphere. Because hydrate breakdown, causing release of methane to the atmosphere, can be related to pressure changes caused by glacial sea-level fluctuations, gas hydrate may play a role in controlling long-term global climate change. 3. It can affect sediment strength, which can initiate landslides on the slope and rise. Gas hydrate apparently cements sediment, and, therefore, it can have a significant effect on sediment strength; its formation and breakdown may influence the occurrence and location of submarine landslides. Such landslides may release methane into the atmosphere, which may affect global climate. Changes in water pressure due to sea level changes may generate landslides by converting the hydrate to gas plus water, causing significant weakening of the sediment, and generating a rise of pore pressure. Conversely, sea-floor landslides can cause breakdown of hydrate by reducing the pressure in sediments. These interacting processes may cause cascading slides, which would result in breakdown of hydrate and release of methane to the atmosphere. Methane Hydrate is the most abundant natural form of clathrate, a unique class of chemical substance in which molecules of one material (in this case, water) form an open solid lattice that encloses, without chemical bonding, appropriately-sized molecules of another material (in this case, methane). The study of natural methane hydrates pose unique technical challenges. They occur only in remote and hostile environments of the arctic and the deep oceans. They are also highly unstable and thus difficult to remove from their natural setting for later lab study. Consequently, scientists employ a wide variety of specially-tailored technologies in their efforts to determine the presence and characteristics of natural methane hydrate. The unusual association of two molecules in a solid substance without bonding has intrigued scientists since the first manufacture of chlorine hydrate almost two centuries ago. However, the subject appeared to be purely academic until solid hydrate was found to be plugging natural gas transmission lines in the 1930s. Then, in the 1960s, naturally-occurring methane hydrate was observed in Siberian gas reservoirs. As the understanding of natural methane hydrate grew, scientists realized that, given the ubiquity of both methane (the common by-product of bacterial breakdown of organic matter) and water in nature, methane hydrate could be present in vast quantities in any environment with suitable pressures and temperatures. Over the past three decades, expeditions to polar regions and deep-water continental shelves all over the globe have consistently returned reports of methane hydrate. Today, the U.S. Geological Survey estimates that methane hydrate may, in fact, contain more organic carbon than all the world's coal, oil, and non-hydrate natural gas combined. The magnitude of this previously unknown global storehouse of methane is truly staggering and has raised serious inquiry into the possibility of using methane hydrate as a source of energy.One technology commonly used to study the deep sea floor, where hydrates occur, is side-scan sonar. Sonar surveys use echo-sounding to provide detailed information on the topography and reflectivity of the sea-floor. The data can provide the initial clues to the presence of near-surface hydrates through identification of anomalous sea-floor features, including slumps and slides, pockmarks, mud diapers, and mud volcanoes. Seismic reflection surveys are the most promising technology for a quick and accurate appraisal of large areas of the deep oceans. Similar to the way a doctor uses a sonogram to see inside the human body, scientists use sound waves to image the structures and properties of the Earth's interior. For decades, industry has conducted seismic surveys to identify promising subsurface geologic structures that may hold oil and gas. Today, geophysicists are working to tailor seismic collection, processing, and interpretation techniques to the investigation of methane hydrate. However, while those investigations continue, important questions about the role of methane hydrate in the environment must be addressed. Recent studies clearly indicate that the global methane hydrate reservoir is in constant flux, absorbing and releasing methane in response to ongoing natural changes in the environment. |
|
||||||||
 |
|||||||||
 |
|||||||||
 |
|||||||||
|
Objectives |
|||||||||
|
1. Providing a fundamental understanding of species flow during hydrate dissociation |
|||||||||
| Computational Modeling | |||||||||
Fluid flow of a dissociating or growing hydrate structure can be modeled as a three-phase, two-component flow system. The three phases are gas, water and hydrate, and the two components are gas and water. Only gas and water are flowing phases; hydrate is a solid phase. There are couple of methods to simulate the natural gas production from hydrate dissociation. The most common method is using a depressurizing well which is drilled into the reservoir. Pressure reduction of a stable hydrate structure will result in gas production from the dissociating hydrate, thus stimulating fluid flow as a function of time t. The flow of gas and water through a hydrate stability zone, on the other hand, will result in the simulation of hydrate growth. The flow of the system can be approximated by coupling fluid-mass conservation with Darcy's law which relates a gradient in pore pressure P and gravity to the rate of fluid flow. The law of mass conservation can be expressed as, (Rate Of Accumulation) = (Rate Transported In) + (Rate Transported Out) + (Rate Of Production) Using FLUENT™ CFD package the dissociation of methane hydrate is found, assuming a two and three dimensional flows in two and three phases through a porous media. An animation of the flow field for the two dimensional case is shown here. The model is still in progress. Future work will be modeling in three dimensional within an actual reservoir. |
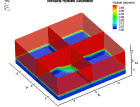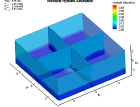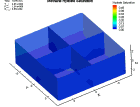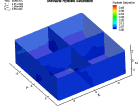 |
||||||||
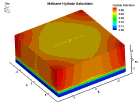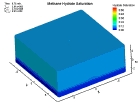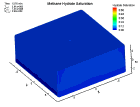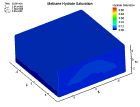 |
|||||||||
 |
 |
||||||||
| Useful Links | |||||||||
|
The Science of Natural Methane Hydrate |
|||||||||
|
Copyright © Kambiz Nazridoust, 2005. |
 back back |
 up up |
|||||||
