|
Effects of Charge
What can make electrostatic interactions more significant?

- Increase the size of the particle.Electrostatic forces as R2 whereas van der Waals forces vary linearly with R.
- Increase the surface-charge density.The critical radius varies as 1/s2.
- Decrease the surface energy/Hamaker constant.Examples include coating a surface with Teflon or zinc stearate.
- Add asperities to the particle.These serve as physical separations that reduce adhesion (Tabor and Fuller (Proc. R. Soc. Lond. A 345, 327 (1975); Schaefer et al. J. Adhesion Sci. Technol. 9, 1049 (1995))
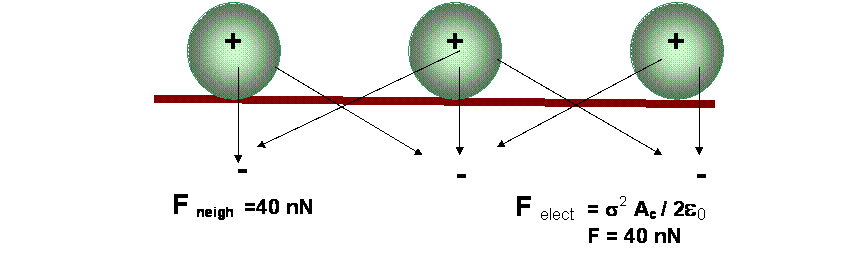
- Add neighboring particles having a similar charge (Goel and Spencer, in Adhesion Science and Technology Part B, L. H. Lee (ed.)).
- Localize charge to specific areas on surface of the particle rather than uniformly distributing it – the so-called “charged patch model” (D.A. Hays, in Fundamentals of Adhesion and Interfaces, D. S. Rimai, L. P. DeMejo, and K. L. Mittal (eds.))
|