 |
Biocomputing
Security System: Concatenated Enzyme-Based Logic Gates Operating as a
Biomolecular Keypad Lock
The computing networks composed of the enzyme-based concatenated logic gates can be used for mimicking various electronic devices. The present paper reports on a novel approach to the assembling of the biomolecular keypad lock using the enzyme-based networking system. The biochemical paths include concert operation of multi-enzyme systems biocatalyzing chain reactions. Taking out one of the biocatalytic unit effectively inhibits the whole chain of the biochemical reactions. This property was used to assemble the enzyme-based biomolecular keypad lock. We designed a model biochemical reaction chain, which included hydrolysis of sucrose to glucose, oxidation of glucose by oxygen to yield hydrogen peroxide, and then oxidation of a synthetic dye, 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), by H2O2 resulting in the formation of a colored product ABTSox. These reaction steps were biocatalyzed by invertase (Inv), glucose oxidase (GOx) and microperoxidase-11 (MP-11) respectively, and they proceeded only in the presence of these enzymes. The enzymes were immobilized on glass beads (1 mm diameter) and used in the immobilized form. The most important feature of the keypad lock system is the dependence of the output signal on the correct order of the input signals. Thus, we performed the experiment when the order of the enzyme-encoded input signals was varied in 6 different combinations. Only one correct order of the input signals (ABC) resulted in the TRUE output signal “1” while all others produced the FALSE output signal “0”. The TRUE output signal “1” can be used to “open” the lock while the FALSE signal “0” can result in the “alarm” signal indicating the wrong password. |
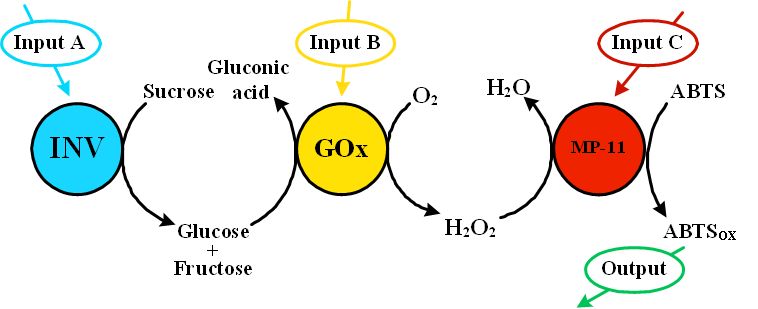 Biocatalytic reactions in the biomolecular keypad lock system. |
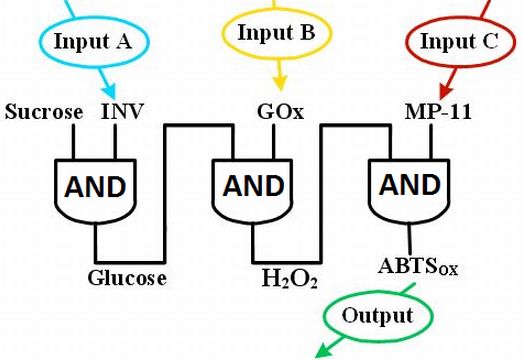 Representation of the biomolecular keypad lock system as a network of three concatenated AND gates. |

The project is mostly performed by PhD student Guinevere Strack.
G. Strack, M. Ornatska, M. Pita, E. Katz, Biocomputing security system: Concatenated enzyme-based logic gates operating as a biomolecular keypad lock. J. Am. Chem. Soc. 2008, 130, 4234-4235 (paper was highligted by ACS and Chem. & Eng. News).
 |
Self-Powered Biomolecular Keypad Lock
Security System Based on a Biofuel Cell
The enzyme-based keypad lock was integrated with a biofuel cell yielding a self-powered biomolecular information security system. The correct “password” introduced into the keypad lock resulted in the activation of the biofuel cell, while all other “wrong” permutations of the enzyme inputs preserved the “OFF” state of the biofuel cell. J. Halámek, T.K. Tam, G. Strack, V. Bocharova, M. Pita, E. Katz, Self-powered biomolecular keypad lock security system based on a biofuel cell. Chem. Commun. 2010, 46, 2405-2407. (paper was highlighted at RSC web
site:
http://www.rsc.org/Publishing/ChemTech/Volume/2010/04/enzymes_power.asp).
|
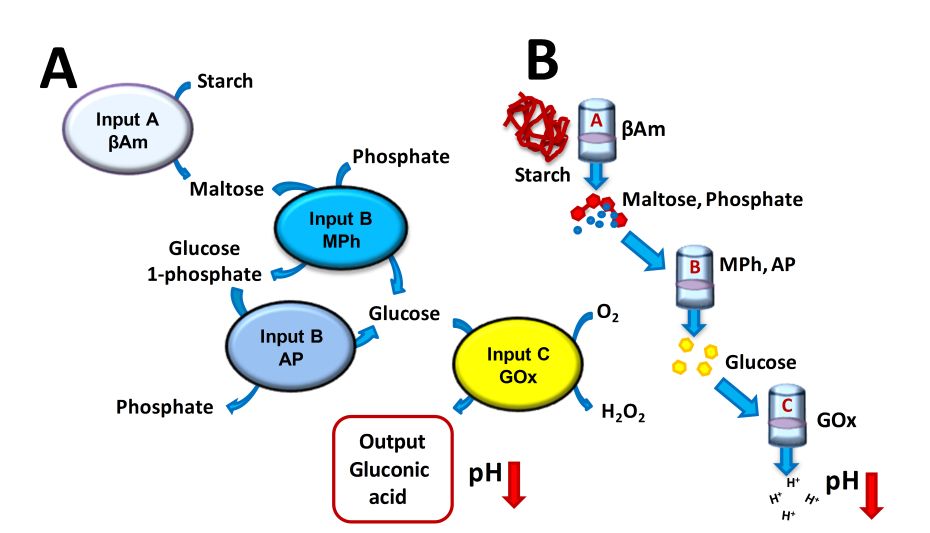 |
 Left:
(A) The biocatalytic cascade triggered by the enzyme inputs when they
are applied in the “correct” A-B-C sequence. (B) The
reaction/separation setup allowing application of the enzyme inputs in
different permutations (shown in the correct order A-B-C).
Top: Logic network corresponding to
the biochemical scheme shown at the left. |
 |
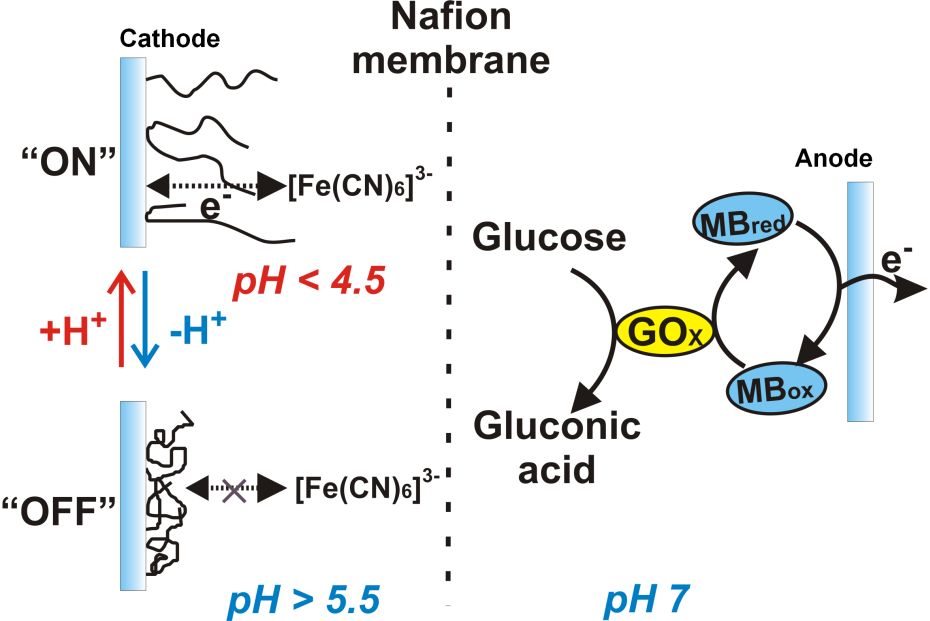
 Left: The
pH-switchable biofuel cell for “reading” the output signals generated
by the keypad lock system. MBox and MBred are the
oxidized and reduced states of methylene blue mediator.
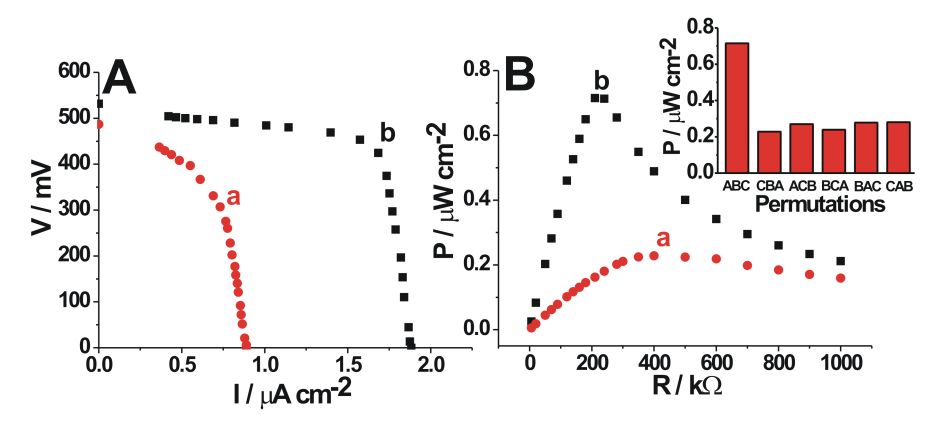
Top: The polarization functions (A) and power output (B) of the biofuel cell obtained at different pH values: a) initial pH 6.7, b) pH 4.2 generated upon A-B-C sequence of the enzyme-inputs. |
  |
Keypad Lock Security System Based on
Immune-Affinity Recognition Integrated with a Switchable Biofuel Cell
The immune-based biorecognition system
mimicking a keypad lock device was integrated with a switchable biofuel
cell resulting in the power output change upon the correct input of the
“password” encoded in the antibody-sequence.
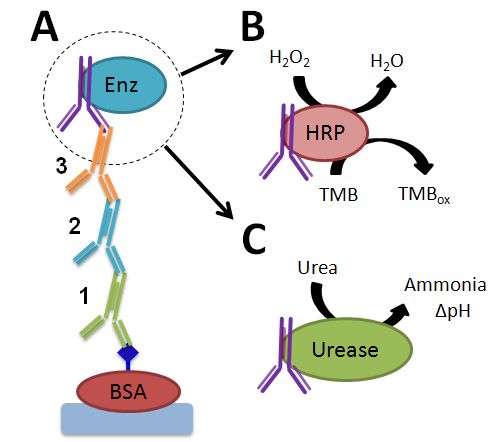
Left: (A) The multi-component immune-recognition system assembled upon application of the antibody-signals in the correct 1,2,3 sequence. (B) The biocatalytic reaction producing the optical output signal upon oxidation of TMB in the presence of HRP bound to the multi-component immune-complex. (C) The biocatalytic reaction producing the pH changes upon formation of ammonia in the presence of urease bound to the multi-component immune-complex. J. Halámek, T.K. Tam, S. Chinnapareddy, V. Bocharova, E. Katz, Keypad lock security system based on immune-affinity recognition integrated with a switchable biofuel cell. J. Phys. Chem. Lett. 2010, 1, 973-977. |
 |
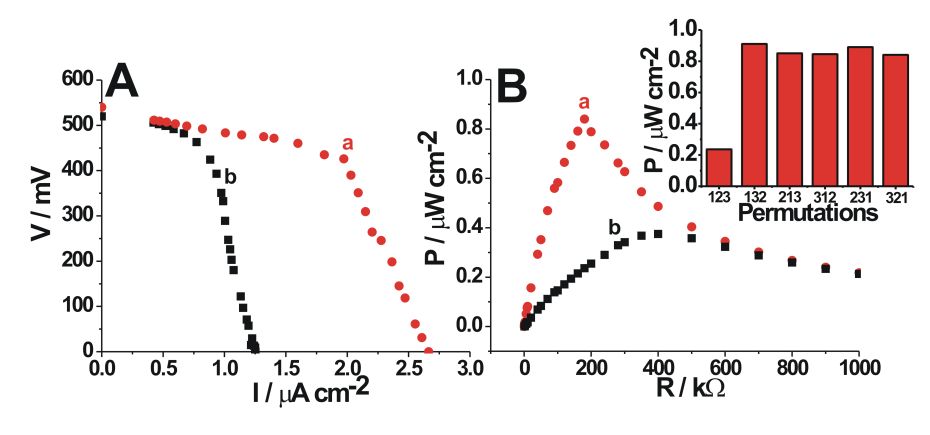 Left: The
biofuel cell with the cathode switchable by pH changes. MBox
and MBred are the oxidized and reduced forms of methylene
blue redox mediator.
Top:
Polarization curves (A) and power output (B) generated by the biofuel
cell: a) in the initial active state (pH 4.2 in the cathodic
compartment), b) after addition of the solution (pH ca. 6.6) generated
by the correct sequence (1,2,3) of the antibody-signals finished with
the urease-labeled antibody. Inset: The power output measured on the
load resistance of 180 kOhm upon application of different
permutations of the antibody-signals and addition of the produced
solution to the cathodic compartment.
|


The project was mostly performed by Dr. Jan Halámek (right) and PhD student Tsz Kin Tam (Ward) (left).
 |
Steganography
and Encrypting Based on Immunochemical Systems
Steganography and encrypting were
demonstrated with immuno-specific systems. IgG-proteins were used as
invisible ink developed with complementary antibodies labeled with
enzymes producing color spots. The information security was achieved by
mixing the target protein-antigens used for the text encoding with
masking proteins of similar composition but having different
bioaffinity. Two different texts were simultaneously encoded by using
two different encoding proteins in a mixture. Various encrypting
techniques were exemplified with the immuno-systems used for the
steganography. Future use of the developed approach for information
protection and watermark-technology was proposed. Scaling down the
encoded text to a micro-size is feasible with the use of
nanotechnology.
K.-W. Kim, V. Bocharova, J.
Halámek, M.-K. Oh, E. Katz, Steganography and encrypting based on
immunochemical systems. Biotechnology and Bioengineering 2011, in press (DOI:
10.1002/bit.23017).
|
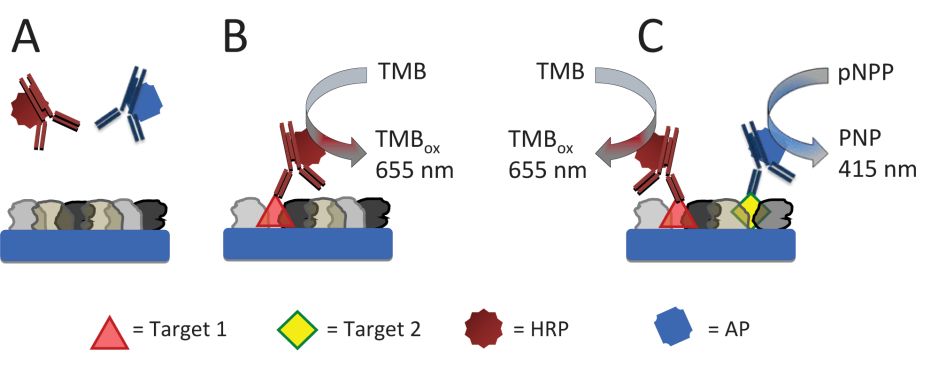 (Scheme
above): )Steganography based on the immuno-specific interactions
resulting in the color-development by the enzyme-labeled complementary
antibody similarly to ELISA technique. A: There is no binding of the
enzyme-labeled antibodies to a mixture of masking proteins. B) The
enzyme-labeled antibody (HRP-labeled anti-mouse IgG) binds to the
corresponding target protein-antigen (mouse anti-human IgG) in the
presence of a mixture of masking proteins, thus resulting in the
biocatalytic oxidation of TMB. C: Two enzyme-labeled antibodies
(HRP-labeled anti-mouse IgG and AP-labeled anti-rabbit IgG) bind to the
corresponding target protein-antigens (mouse anti-human IgG and rabbit
anti-rat IgG) in the presence of a mixture of masking proteins, thus
resulting in the biocatalytic oxidation of TMB and formation of PNP.
Inset: Images show protein-modified wells corresponding to the
biorecognition systems shown schematically above.
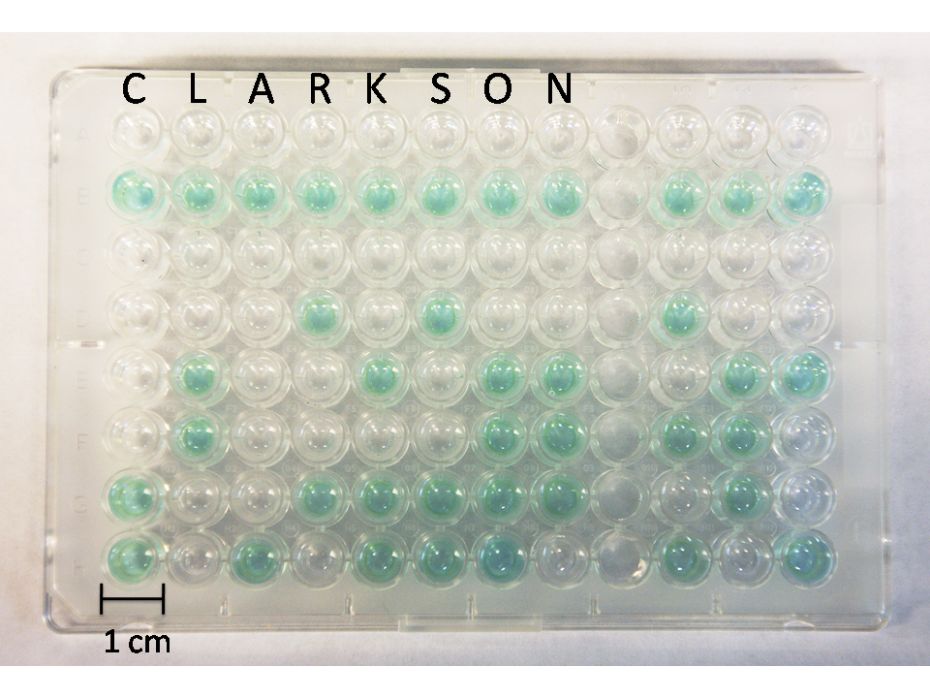
(Figure at right): Absorbance developed on spots composed of: A: mouse anti-human IgG (target protein), goat anti-cat IgG (masking protein) and donkey anti-chicken IgG (masking protein); B: only masking proteins without the target protein-antigen, after their treatment with HRP-labeled anti-mouse IgG (antibody complementary to the target protein-antigen) and further reaction with H2O2 and TMB. The experiment was performed on an ELISA plate. |
 |

The experiments with the steganography and encrypting systems were performed by PhD-internship student Kyung-Woo Kim.

Updated
on February 21, 2011