|
Introduction
Particles greater than 5 – 10 µm are usually removed by the upper
respiratory system. But particles smaller than 5 µm can penetrate
deep into the lung and become a health hazard. Typical ranges of values
for aerosol parameters for aerosols are listed in Table 1. The corresponding
values for air (N2 ) are also shown in this table for comparison.
Table 1: Atmospheric Aerosol Parameters
| |
Aerosols |
Air |
Number Density
(Number/cm3) |
100-105 |
1019 |
| Mean Temperature (K) |
240-310 |
240-310 |
| Mean Free Path |
Greater than 1m |
0.06µmM |
| Particle Radius |
0.01 – 10 µm |
2x104µm |
| Particle Mass (g)
| 10-18 - 10-19 |
4.6x10-23 |
Particle Charge
(in Elementary Charge Units) |
0 – 100 |
Weakly Ionized
Single Charge |
The important dimensionless groups relevant to the motion of aerosols
are listed in Table 2.
Table 2: Dimensionless Groups
| Knudsen Number |
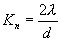 |
| Mach Number |
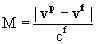 |
| Schmidt Number |
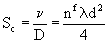 |
| Brown Number |
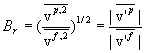 |
| Reynolds Number |
 |
Here are definitions for the following symbols:
| λ = Mean Free Path |
ν = Kinematic Viscosity |
| d = Particle Diameter |
D = Diffusivity |
vp = Particle Velocity |
v’ = Thermal Velocity |
vf = Fluid (Air) Velocity |
n = Number Density |
| cf = Speed of Sound |
|
Here, superscript "f" refers to fluids and superscript
"p" refers to particles.
In these equations the root mean fluctuation velocity is given by
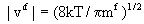
and 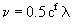 . .
The mean free path of the gas is given as
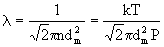
Here n is the gas number density, dm is the gas molecule (collisional)
diameter, k=1.38x10-23 J/K is the Boltzman constant, P is pressure,
and T is temperature. For air, dm = 0.361nm and
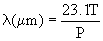 , ,
P is in Pa, and T is in K. Table 3, below,
illustrates these Aerosol Characteristics.
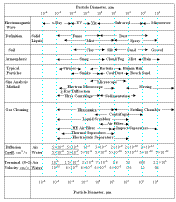
|