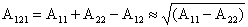|
London-van der Waals Force
London-van der Waals Surface Energy Between Particles
The London-van der Waals surface energy and force between two
spherical particles of diameters
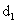 and and
 as show
in Figure 4 was evaluated by Hamaker (1937). The corresponding
surface energy is given as as show
in Figure 4 was evaluated by Hamaker (1937). The corresponding
surface energy is given as
 |
(13) |
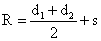 |
(14) |
is the distance between particles center and s is the separation distance between surfaces.

Figure 4. Schematics of contact of two dissimilar spheres.
For equal size particles,  , ,
 , and , and
 |
(15) |
As noted before, A is typically of the order of 10-19 to 10-21 J and depends
on the properties of particles (of composition 1) and suspending medium
(composition 2). Accordingly, the effective Hamaker constant is given by
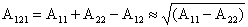 |
(16) |
| 

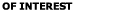

























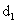 and
and
 as show
in Figure 4 was evaluated by Hamaker (1937). The corresponding
surface energy is given as
as show
in Figure 4 was evaluated by Hamaker (1937). The corresponding
surface energy is given as
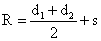
 ,
,
 , and
, and