|
London-van der Waals Force
The London-van der Waals force, which is generally attractive in nature,
is a short range force and decays rapidly to zero away from a surface.
The origin of the London-van der Waals force lies in the instantaneous dipole
generated by the fluctuation of electron cloud surrounding the nucleus of
electrically neutral atoms. For a spherical particle of diameter d near
a flat surface, the interaction energy is given by:
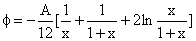 , , |
(1) |
where x=z/d and z is the distance of the sphere from the surface and A is
the Hamaker constant. As the particle approaches the surface,
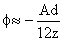 ,
as ,
as 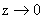 |
(2) |
Thus, the energy becomes infinite for z = 0. Hence, the surface acts as a
perfect sink for aerosol diffusion. The range of operation of the van der Waals
force may be estimated by comparing the thermal energy with
 . Values of Hamaker
constant A are in the range of . Values of Hamaker
constant A are in the range of  to
to 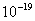 J. Thus, J. Thus,
 for for
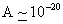 . . |
(3) |
In Table 1 values for van der Waals force for a number of materials are
listed and the values of van der Waals force is compared with the Stokes drag
force acting on a particle that is moving with a velocity of 1 m/s in aid and
in water. It is seen that the van der Waals force in air is comparatively
larger than that in water. Furthermore, van der Waals force is much
larger that the drag force. The ratio of the van der Waals force to drag
force in water is generally less than that in air.
|






















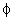 . Values of Hamaker
constant A are in the range of
. Values of Hamaker
constant A are in the range of  to
to 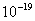 J. Thus,
J. Thus,


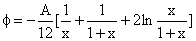 ,
,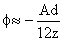 ,
as
,
as 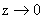
 for
for
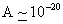 .
.