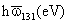|
London-van der Waals Force
Hamaker Constants for Dissimilar Materials
Table 3. Values of Lifshitz -van der Waals Constant
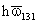 for some materials (Visser, 1976).
for some materials (Visser, 1976).
|
Homogenous Combinations
|
|
|
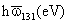
|
|
Combinations |
Vacuum |
Water |
|
Au-Au |
14.3 |
9.85 |
|
Ag-Ag |
11.7 |
7.76 |
|
Cu-Cu |
8.03 |
4.68 |
|
Diamond-Diamond |
8.6 |
3.95 |
|
Si-Si |
6.76 |
3.49 |
|
Ge-Ge |
8.36 |
4.66 |
|
MgO-MgO |
3.03 |
0.47 |
|
KCl-KCl |
1.75 |
0.12 |
|
KBr-KBr |
1.87 |
0.18 |
|
KI-KI |
1.76 |
0.20 |
|
Al2O3-Al2O3 |
4.68 |
1.16 |
|
CdS-CdS |
4.38 |
1.37 |
|
H2O-H2O |
1.43 |
- |
|
Polystyrene-Polystyrene |
1.91 |
0.11 |
Table 4. Values of Lifshitz -van der Waals Constant
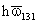 for some materials (Visser, 1976).
for some materials (Visser, 1976).
|
Heterogeneous Combinations
|
|
|
|
|
Combinations |
Water |
Polystyrene |
|
Au-Ag |
- |
8.27 |
|
Au-Cu |
6.41 |
5.93 |
|
Au-Diamond |
6.11 |
5.45 |
|
Au-Si |
5.32 |
4.70 |
|
Au-Ge |
6.50 |
5.93 |
|
Au-MgO |
1.99 |
1.25 |
|
Au-KBr |
0.73 |
0.0 |
|
Au-Al2O3 |
- |
2.60 |
|
Au-CdS |
- |
2.65 |
|
Au-Polystyrene |
0.72 |
- | |


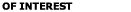




















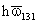 for some materials (Visser, 1976).
for some materials (Visser, 1976).
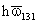 for some materials (Visser, 1976).
for some materials (Visser, 1976).